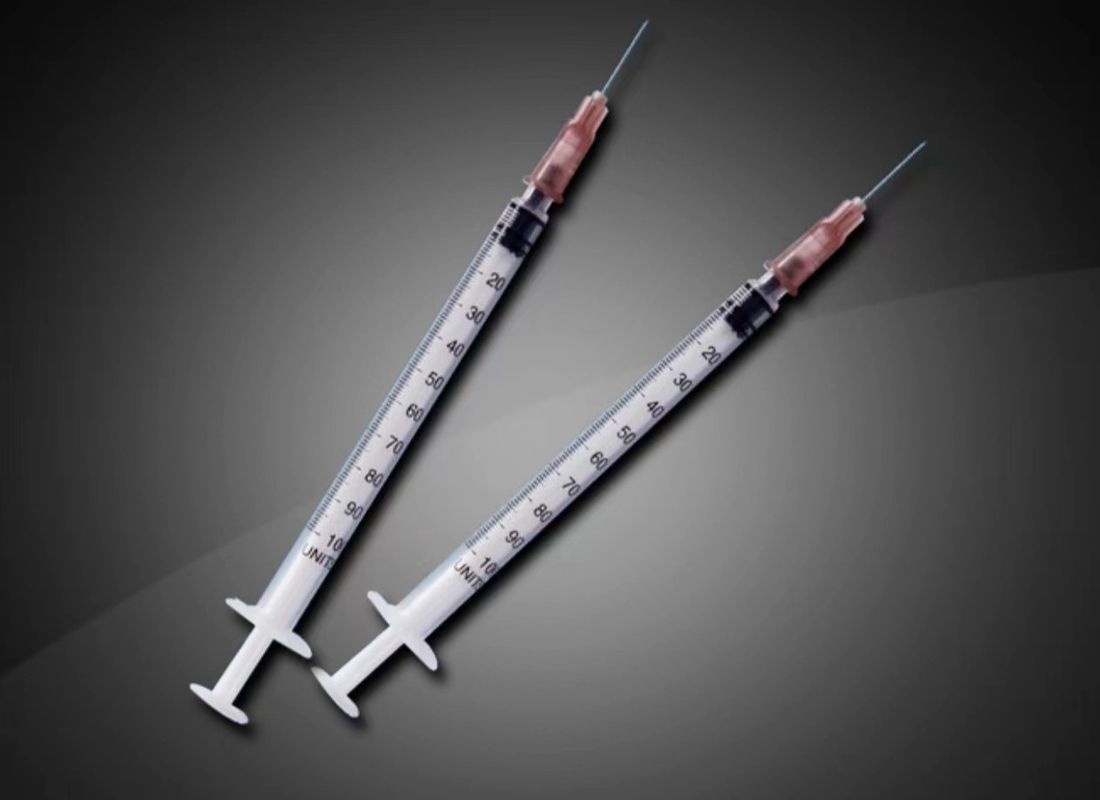
Fast Shipping Sterile Injection Disposable Bacilluus Tuberculiluus Syringe BCG Quality Certification CE
-
Highlight
Fast Shipping Disposable Sterile Syringe
,CE Disposable Sterile Syringe
,Bacilluus Tuberculiluus Disposable Sterile Syringe
-
NameBacilluus Tuberculiluus Syringe
-
MaterialMedical Grade PP
-
Instrument ClassificationClass II
-
PropertiesInjection & Puncture Instrument
-
Unit PackingPE/Blister
-
OEMAvilable
-
CertificateCE ISO
-
Size0.05-1ml
-
Place Of OriginChina
-
Place of OriginChina
-
Brand NameHenan Aile
-
CertificationCE
-
Model Number0.05-1ml
-
Minimum Order Quantity70000pcs
-
PriceNegotiable
-
Packaging DetailsSingle in paper-plastic packaging ,200pcs/box
-
Delivery Time25-30 days
-
Payment TermsL/C, T/T
-
Supply Ability30000000 pcs per month
Fast Shipping Sterile Injection Disposable Bacilluus Tuberculiluus Syringe BCG Quality Certification CE
Fast Shipping Sterile Injection Disposable Bacilluus Tuberculiluus Syringe (BCG), Quality Certification CE
- Blister pack/PE bag.
- High barrel clarity. Easy readability, accurate and scale markings.
- Store in relative humidity which not surpass 80% in dry room, non corrosiveness gas.
| Cat.NO. | Specification |
| AL111711 | Tuberculin bacillus syringe |
| AL111711 | Capacity:0.05ml, 0.1ml, 0.2ml, 0.3ml, 0.5ml, 1ml |
| AL111711 | With or without fixed needle, needle size:26G-30G |
| AL111711 | 3parts, luer slip |
| AL111711 | Non-toxic, non-pyrogenic, for precise dosage |
![]()
3.More details on the intended use and safety features of the disposable Bacillus Tuberculosis syringe
As of my last update in September 2021, there is no specific information available about a syringe designed specifically for Bacillus tuberculosis. However, I can provide some general information on the intended use and safety features that might be considered for a syringe used in the context of tuberculosis testing and treatment:
Intended Use:
- Tuberculosis Testing: The syringe may be designed for administering Mantoux tuberculin skin tests to check for tuberculosis infection.
- Medication Administration: It could be used for administering medications used in the treatment of tuberculosis.
Safety Features:
- Sterility: The syringe should be sterile to prevent infections and cross-contamination during testing and treatment.
- Precision: Designed for accurate and precise dosing to ensure the correct amount of medication or tuberculin is administered.
- Single-Use: Typically disposable for single-use to prevent the risk of contamination and ensure patient safety.
- Needle Safety: May include safety features like needle guards or mechanisms to prevent needle-stick injuries after use.
- Material: Constructed from medical-grade materials to meet safety and quality standards.
Additional Considerations:
- CE Certification: Compliance with European standards for medical devices could be important for ensuring safety and quality.
- Clear Markings: Clear volume markings on the syringe for accurate dosing.
- Ease of Use: Designed for easy handling and administration by healthcare professionals.
For specific information regarding a "Disposable Bacillus Tuberculosis Syringe," it would be advisable to consult with healthcare professionals or medical device manufacturers for the most up-to-date and accurate details.
4.Is BCG vaccine injected subcutaneously or intradermally? What should be noted when using?
When administering BCG vaccine, intradermal injection should be performed at the lower edge of the deltoid muscle on the outer side of the upper arm, and subcutaneous or intramuscular injection is strictly prohibited.
Bacillus Calmette Guerin (BCG) is a live vaccine made of attenuated bovine tuberculosis bacillus. It can be inoculated within 24 hours of birth to prevent tuberculosis. Generally, the site of vaccination with BCG vaccine is the middle and lower edge of the lateral deltoid muscle in the upper arm. If injected on the shoulder or above the deltoid muscle, it is likely to cause the formation of raised scars in the local area. Injecting into the middle and lower margin of the deltoid muscle can reduce the occurrence of such situations.